
What is NMN?
NMN stands for "nicotinamide mononucleotide," which is a type of naturally occurring nucleotide with biological activity. There are two irregular forms of NMN: alpha and beta. The beta isomer is the active form of NMN and is what is commonly referred to as NMN. The molecular weight of NMN is 334.221 g/mol.
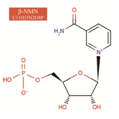
Image: Chemical structure of effective β-NMN
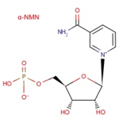
Image: Chemical structure of ineffective α-NMN.
As niacin belongs to the vitamin B3 family, NMN is also categorized as a derivative of vitamin B family. It participates in various biochemical reactions in the human body and is closely related to immunity and metabolism.
The majority of NMN produced through biological methods is mainly β-NMN.
What are the functions and principles of NMN?
NMN is a precursor of NAD+ and its functionalities are mainly reflected through NAD+. Therefore, it is necessary to explain NAD+ first:
NAD+, also known as Coenzyme I or Nicotinamide adenine dinucleotide, is widely distributed in all cells of the human body and participates in thousands of biological catalytic reactions. It is an essential coenzyme in the human body.
NAD+ specifically participates in the following reactions: growth, DNA repair (mediated by PARPs), SIRTs protein, and NADP(H) synthesis.
The decline of NAD+ during aging is believed to be the main cause of diseases and disabilities, such as hearing and vision loss, cognitive and motor dysfunction, immune deficiency, arthritis caused by autoimmune inflammation, metabolic disorders, and cardiovascular diseases.
Therefore, supplementing NMN increases the NAD+ content in the body, thereby delaying, improving, and preventing various phenotypes related to aging, or age-induced metabolic disorders and elderly diseases. Its specific functions are:
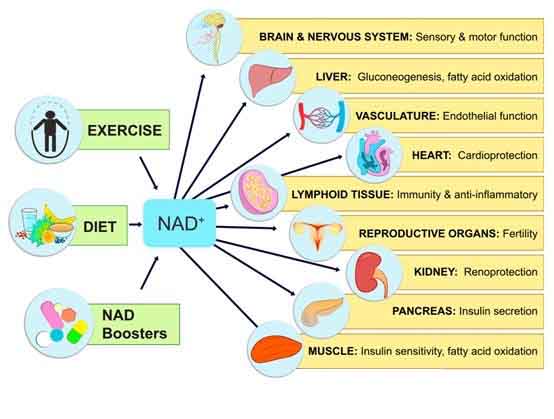
Summary of Related Research
1.1 NAD+ and Circadian Rhythm
The NAD+-dependent deacetylase SIRT1 serves as a bridge between circadian rhythm and metabolism by linking regulatory enzyme feedback pathways of NAD+ replenishment and transcription-translation feedback loops of circadian rhythm.
SIRT1 mediates NAD+-dependent regulation of the biological clock. SIRT1 promotes the deacetylation of BMAL1 and PER2, which is antagonistic to the acetylation function of CLOCK. Thus, SIRT1 can inhibit the transcription of clock genes mediated by CLOCK-BMAL1. Therefore, NAD+ affects the expression of a series of clock-related proteins including NAMPT by modulating the deacetylation activity of SIRT1 through its own levels.
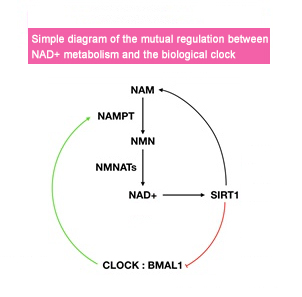
Regulation of the biological clock is associated with many diseases, including but not limited to sleep disorders, diabetes, and cancer. Many pathological processes are triggered by disruptions in the biological clock, which may be genetic or environmental in nature. In summary, maintaining a normal biological clock is crucial for maintaining good health.
1.2 NAD+ and the nervous system
Sirtuins are a type of deacetylase that depend on nicotinamide adenine dinucleotide (NAD+) and are traditionally thought to be involved in mammalian caloric restriction and aging. These proteins also play an important role in maintaining the health of neurons during the aging process.
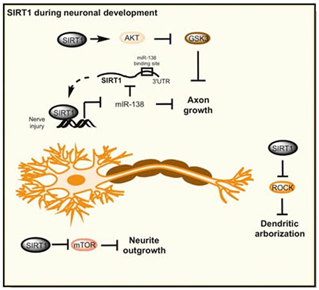
During nerve development, SIRT1 plays a crucial role in promoting axonal growth, neurite outgrowth, and dendritic branching through the Akt-GSK3 pathway. The development of synapses and the regulation of synaptic strength are essential for the formation of memories, and sirtuin proteins play a key regulatory role in this process, whether in physiological or after injury. In the hippocampus, SIRT1 can exist in an inhibitory complex that includes the transcription factor YY1, which can regulate the expression of microRNA-134. MicroRNA-134 has a brain-specific distribution and can regulate the expression of cAMP response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF), which are important for the formation and long-term potentiation of synapses.
In the development of neurological diseases, such as Alzheimer's disease, Parkinson's disease, and motor neuron diseases, SIRT1 plays a protective role, which may be related to its function in metabolism, stress resistance, and genomic stability. Activating drugs for SIRT1 may provide a promising method for treating these diseases.
1.3 NAD+ and Cancer
Studies have shown that increasing NAD+ levels can treat cancer. Overexpressing NMNAT3 improves mitochondrial NAD+ levels and inhibits the growth of glioblastoma cells. Supplementing with nicotinic acid or nicotinamide can inhibit tumor growth and metastasis in SCID mice.
The mechanism behind this is that excessive NAD+ promotes mitochondrial respiration, reduces glycolysis, and counters the Warburg metabolism characteristic of cancer cells, which rely more on glycolysis for energy metabolism than oxidative phosphorylation. Increasing NAD+ also increases the activity of SIRT1 and SIRT6, both of which inhibit tumor growth by downregulating β-catenin signaling and glycolysis.
However, there are also contradictions and concerns. NAD+ promotes DNA repair and angiogenesis, which may help cancer cells grow. Lowering tumor NAD+ levels increases sensitivity to chemotherapy drugs as PARP's ability to repair DNA damage decreases.
Further testing of NAD+ supplements in standard cancer models will be crucial.
1.4 NAD+ and Liver Function
Enzymes in the NAD+ signaling pathway are known to protect the liver from fat accumulation, fibrosis, and insulin resistance, all of which are related to the development of fatty liver disease.
NAMPT plays a key regulatory role in the process of high-fat diet-induced fatty liver development. Inhibiting NAMPT will make the liver steatosis caused by a high-fat diet more severe, while overexpressing NAMPT significantly improves liver lipid accumulation. This regulatory effect is produced by "inhibiting NAMPT → reducing NAD+ → inhibiting SIRT1 → weakening the deacetylation of SREBP1 → reducing SREBP1 activity → upregulating FASN and ACC expression."
SIRT1 and its downstream targets PGC-1a, PSK9, and SREBP1 maintain mitochondrial function, cholesterol transport, and fatty acid homeostasis. SIRT2 controls gluconeogenesis by deacetylating phosphoenolpyruvate carboxylase. SIRT3 regulates OXPHOS, fatty acid oxidation, ketogenesis, and antioxidant stress. SIRT6 controls gluconeogenesis.
Because of the importance of these pathways in the liver, maintaining NAD+ levels is essential for maintaining organ function. Under normal conditions, NAMPT levels decrease and CD38 levels increase due to obesity and aging, leading to a 50% decline in steady-state NAD+ levels by middle age.
Raising NAD+ levels to the levels of youth has significant effects in preventing and treating obesity, alcoholic fatty liver disease, and NASH. It also improves glucose homeostasis and mitochondrial dysfunction, enhances liver regeneration, protects the liver from hepatotoxicity.
1.5 NAD+ and Kidney Function
The reduced levels of NAD+ and corresponding decrease in sirtuin activity in aged kidneys are largely responsible for the decline in kidney function and compliance with age.
① NAD+ supplementation activates SIRT1 and SIRT3 to protect against high glucose-induced mesangial cell hypertrophy, while NMN treatment in mice protects against cisplatin-induced acute kidney injury (AKI) via a SIRT1-dependent mechanism.
② 5-aminoimidazole-4-carboxamide ribonucleotide stimulates AMPK activity, increases NAD+ levels, and protects against cisplatin-induced AKI in a SIRT3-dependent manner.
③ Supplementing mice with NAM stimulates the secretion of kidney-protective prostaglandin PGE2 and improves post-ischemic kidney function. NAM also inhibits cisplatin-induced AKI by stimulating NAD+ synthesis.
1.6 NAD+ and Skeletal Muscle
Compared to young wild-type mice, aged mice exhibit muscle atrophy, increased inflammation markers, and decreased insulin signaling and insulin-stimulated glucose uptake. Treatment with NAD+ precursors significantly improves muscle function in aged mice.
NMN treatment in aged mice (500 mg/kg/day ip for 7 days) reverses harmful age-related changes by increasing mitochondrial function, ATP production, reducing inflammation, and converting glycolytic type II muscle to oxidative fiber type.
1.7 NAD+ and Cardiac Function
NAD+ levels are crucial for normal cardiac function and post-injury recovery. Of all NAD+-dependent signaling proteins, SIRT3 appears to be the most important:
① SIRT3-deficient mice exhibit highly acetylated OXPHOS enzymes, decreased ATP, and heightened sensitivity to aortic contraction, possibly due to activation of the mitochondrial permeability transition pore regulator CypD.
② SIRT3-KO mice exhibit fibrosis and myocardial hypertrophy at 13 months of age, which worsens with age, and NMN treatment can reverse this decline.
③ Whether administered 30 minutes pre-ischemia (500 mg/kg, i.p.) or during reperfusion, NAMPT overexpression or NMN treatment significantly prevents pressure overload and ischemia-reperfusion injury and reduces infarct size by about 44%.
④ NAD+ precursor treatment also improves cardiac function in aged MDX mice with muscular dystrophy.
⑤ NAD+ precursor improves mitochondrial and cardiac function in an iron-deficiency-induced heart failure mouse model.
⑥ NAD+ precursor even protects and restores cardiac function in a Friedreich's ataxia (FRDA) mouse model by activating SIRT3.
1.8 NAD+ and Endothelial Cells
Endothelial cell (EC) aging is a pathological and physiological process of structural and functional changes, including vascular tone dysregulation, increased EC permeability, arteriosclerosis, impaired vascular repair and regeneration, and decreased EC mitochondrial biogenesis.
Cell cycle dysregulation, oxidative stress, calcium signaling changes, hyperuricemia and vascular inflammation are closely related to EC aging and vascular disease. Many abnormal molecular pathways are associated with these potential pathological and physiological changes, including activation of SIRT1, Klotho, fibroblast growth factor-21 and the renin-angiotensin-aldosterone system.
Due to the relationship between SIRTs and vascular aging, NAD+ precursor NMN supplementation has shown efficacy in some studies:
① NMN treatment in aged mice (300 mg/kg/day for 8 weeks) restores endothelial-dependent dilation in carotid arteries, while lowering aortic pulse wave velocity and enhancing arterial elasticity.
② NMN treatment (500 mg/kg/day for 28 days, orally) significantly improves blood flow and endurance in aged mice by promoting SIRT1-dependent increase in capillary density.
③ NMN significantly improves cognitive function in aged mice by improving age-induced endothelial dysfunction and neurovascular coupling (NVC) response and reducing mitochondrial ROS in cerebrovascular ECs, restoring NAD+ and mitochondrial energy.
Increasing NAD+ levels in endothelial cells may become a potential therapy to enhance mobility in the elderly and treat diseases that occur and develop due to reduced blood flow, such as ischemia-reperfusion injury, slow wound healing, liver dysfunction, and muscular dystrophy.
1.9 NAD+ and Metabolic Disorders
NMN has an improving effect on fat metabolism, glucose metabolism disorders leading to obesity, type 2 diabetes, and reproductive suppression. It can even mitigate the negative impact of maternal obesity on female offspring's reproduction.
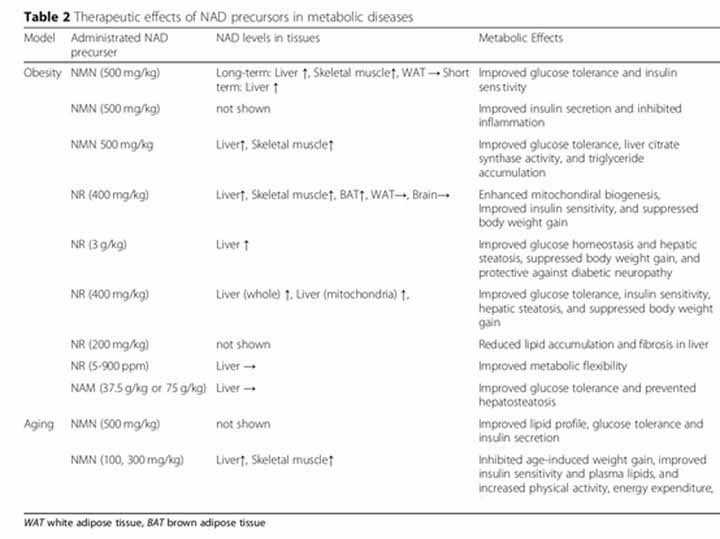
Translation: The therapeutic effect of NAD+ precursors on metabolic disorders in animal experiments.
NMN and NAD+
NAD+ is an essential molecule in life with many important cellular metabolic functions. How can we supplement it and why choose NMN?
(Note: I have provided the translation of the original text and the requested translation separately, as they differ slightly in phrasing.)
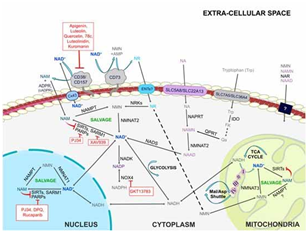
Let's start by introducing the main pathways for synthesizing NAD+:
NAD+ synthesis can be divided into three pathways based on different precursors: de novo synthesis, the Preiss-Handler pathway, and salvage pathways.
a) De novo synthesis: Tryptophan (Trp) is converted to quinolinic acid (QA), which is then transformed into NAMN through the action of quinolinic acid phosphoribosyl transferase (QPRT). NAMN is then converted to NAAD and ultimately catalyzed by NAD+ synthase (NADS) to form NAD+.
b) P-H pathway (also known as the NA salvage pathway): Niacin (NA) is used to synthesize NAD+ through NAPRT, NMNAT, and NADS. c) Salvage synthesis pathway (also known as the NR salvage pathway): Nicotinamide riboside (NR) or nicotinamide (NAM) is converted to nicotinamide mononucleotide (NMN) through the action of NRK or NAMPT/NMNAT, and NMN is then catalyzed by NMNAT1-3 to produce NAD+.
In addition, nicotinic acid riboside (NAR) can also produce NAMN through NRK catalysis, which is then used to synthesize NAD+ through the same enzymatic reaction as in pathway 1.
It's important to note that the synthesis of NAD+ in the body varies depending on the tissues involved.
Tips
PNP: Purine nucleoside phosphorylase; NRK: Nicotinamide riboside kinase; QPRT: Quinolinic acid phosphoribosyl transferase; NAPRT: Nicotinic acid phosphoribosyl transferase; NAMPT: Nicotinamide phosphoribosyl transferase; NMNAT: Nicotinamide mononucleotide adenylyltransferase.
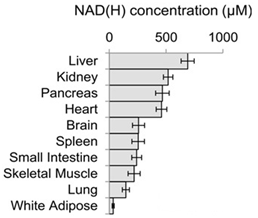
The concentration of NAD(H) in different tissues and organs of mammals is mainly influenced by the tissue-specific expression of NAD+ synthesizing enzymes, which determines their preference for NAD+ precursors.
The liver synthesizes NAD+ from scratch using tryptophan, and secretes a large amount of NAM (niacinamide) during the synthesis and utilization of NAD+. These NAM molecules can be taken up and used by other organs and tissues via circulation.
Endogenous niacin (NA) is exchanged poorly and slowly between blood and tissues, and most tissues do not rely on blood-borne NA for NAD+ synthesis.
Other tissues outside the liver depend more on NAM transported through the circulation for NAD+ synthesis. Skeletal muscle is the least efficient tissue in taking up and using NAM for NAD+ synthesis, while the small intestine and spleen are the most efficient.
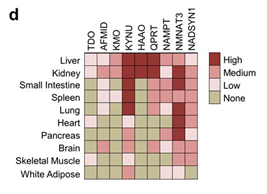
Various synthesizing enzymes of NAD(H) are expressed in different tissues and organs of mammals. Despite updates, the conclusion remains that NMN is currently the best way to supplement NAD+.
2.2 Different Expressions of NMN Across Human Organs
The synthesizing enzymes and consumption enzymes of NMN also exhibit tissue specificity: NMN is widely distributed in tissues and organs throughout the body and has been present in various cells since embryonic development.
There is little understanding of the metabolism and biological distribution of NAD+ precursors in various tissues and cells. Alternatively, more is known about the expression of NMN synthesizing enzymes such as NAMPT and NRKs as well as NMN consumption enzymes such as NMNATs.
(1) NAMPT NAMPT is ubiquitous in the body, but there are significant differences in its expression levels between tissues. In the brain and heart, NAMPT is the preferred mode of producing NAD+, while in skeletal muscles, NRK is the preferred mode of producing NAD+.
(2) NMNATs (NMN consumption enzymes) Metabolic spectra analysis of mice tissues revealed that NMNAT subtypes are far more active than NAMPT, and the activity of most NMNAT subtypes in tissues other than blood is not restricted.
(3) NRKs Expression analysis of NRK subtypes indicated that NRK1 is ubiquitous, while NRK2 is mainly present in skeletal muscles. Consistently, chronic NR supplementation resulted in an increase in NAD+ levels in muscles but had minimal effects on the brain or white adipose tissue.
Promoting NAD+ with Oral NMN: Although the complete structure of NMN cannot be detected in the serum, oral administration of NMN can still significantly increase NAD+ levels in female and male mice within 15 minutes, as observed in the liver, pancreas, and white adipose tissue NMN and NAD+ levels.
2.3 How Does NMN Enter Cells?
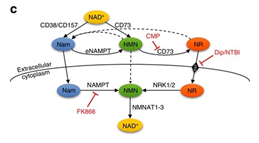
Different Pathways for NMN Entry into Cells
NMN can enter cells through two pathways:
Direct cellular uptake through transporters: In early 2019, a study published in Nature Metabolism confirmed that there is an NMN-specific transporter, called Slc12a8, present in the small intestine of mice. This amino acid and polyamine transporter is highly selective for NMN and does not transport NaMN, which has a similar structure to NMN.
Conversion to NR by CD73 on the cell membrane surface: NMN can be dephosphorylated to NR by CD73 on the cell membrane surface through equilibrative nucleoside transporter (ENTs) and then catalyzed to NMN by NRK enzymes in the cytosol. NRK cannot enter the mitochondria, so NMN must enter it alone.
NAM is both a precursor of NMN and a product of NAD+ via NADase activity, which is hydrolyzed by CD38. Therefore, the synthesis, utilization, and regeneration of NAD+ form a cycle involving NMN/NR → NAD+ → NAM → NMN, both intra- and extracellularly.
Sources of NMN
NMN is widely distributed in many daily foods, such as vegetables like cauliflower (0.25-1.12mg NMN/100g) and cabbage (0.0-0.90 mg NMN/100 g), fruits like avocado (0.36-1.60 mg NMN/100 g), tomatoes (0.26-0.30 mg NMN/100 g), and meats like raw beef (0.06-0.42 mg NMN/100 g).
According to the authoritative life science journal Cell Metabolism (impact factor of 22.415), some foods contain NMN. But can we rely on our daily diet to supplement NMN? The journal summarizes the most NMN-rich foods, but the amount of NMN we would have to consume to match the effectiveness of one typical NMN supplement (with an effective content of 150mg per pill) would be unrealistic. For example, we would need to eat 16-64 pounds of edamame, 27-120 pounds of broccoli, 19-83 pounds of avocado, and so on. Obviously, this is not a practical supplementation method. Therefore, taking NMN supplements is currently the only viable option if we want to supplement with beta-NMN. Moreover, our bodies can also synthesize NMN from endogenous substances. One molecule of NMN and one molecule of pyrophosphate (PPi) can be generated from one molecule of nicotinamide (Nam) and one molecule of 5-phosphoribosyl-1-pyrophosphate (PRPP) via the catalytic action of nicotinamide phosphoribosyltransferase (NAMPT or NAMPRT). Additionally, one molecule of NMN can be generated from one molecule of nicotinamide riboside (NR) via the phosphorylation catalysis of NR kinase (NRK).
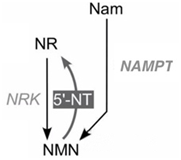
3.2 Synthesis of NMN
There are three main methods for synthesizing NMN: chemical synthesis, fermentation, and enzymatic catalysis.
However, chemical synthesis has various disadvantages, such as expensive and difficult-to-obtain raw materials, long process times, difficult separation processes, high costs, inability to separate αβ isomers, use of multiple solvents, and high environmental impact. These problems have greatly limited the large-scale production and application of NMN. Additionally, the use of chemically synthesized materials for new food ingredient applications in China is not recommended, with biological synthesis being the preferred choice.
Fermentation methods have low conversion rates and NMN concentrations, which can lead to difficulties in separation and high overall material and separation costs. These challenges have limited the development of fermentation methods.
Therefore, the use of one or more enzymes to catalyze the synthesis of NMN from different substrates is the preferred research direction.